A oxyhydrogen fuel cell has a battery using oxygen as an oxidant, hydrogen as a fuel, and then various chemical reactions of the fuel to convert the generated chemical energy into electrical energy. Hydrogen-oxygen fuel cells have many advantages such as large capacity, high specific energy, high conversion efficiency and wide power range. The oxyhydrogen fuel cell is very different from the general battery. Generally, the active material of the battery is stored inside the battery, so the amount of the active substance stored determines the capacity of the battery. The active material of the fuel cell can be continuously input. Today, Xiaobian will introduce some knowledge about oxyhydrogen fuel cells.
Classification of oxyhydrogen fuel cellsHydrogen-oxygen fuel cells are classified into three types: ion membrane, bacon type and asbestos membrane according to the structure and working mode of the battery.
1. Ion-membrane oxyhydrogen fuel cell
An acidic fuel cell using a cation exchange membrane as an electrolyte, a perfluorosulfonic acid membrane is modernly used. When the battery is discharged, water is generated at the oxygen electrode, and water is sucked out through the wick. The battery operates at room temperature, has a compact structure and is light in weight, but the internal resistance of the ion exchange membrane is large, and the discharge current density is small.
2, bacon fuel cell
It is an alkaline battery. The hydrogen and oxygen electrodes are double-layer porous nickel electrodes (the inner and outer diameters are different), and platinum is used as a catalyst. The electrolyte is 80% to 85% caustic potash solution, solid at room temperature, and liquid at battery operating temperature (204 to 260 ° C). This battery has a high energy utilization rate, but it consumes a large amount of electricity, and it takes a long time to start and stop (24 hours for starting and 17 hours for stopping).
3. Asbestos membrane fuel cell
Also an alkaline battery. The hydrogen electrode is made of porous nickel plate with platinum and palladium catalyst. The oxygen electrode is a porous silver pole piece. The two electrodes are sandwiched with an asbestos film containing 35% caustic potash solution, and then pressed with a slotted nickel plate on the two plates as a set. The flow device constitutes a gas chamber and is packaged into a single battery. Water is generated on the side of the hydrogen electrode during discharge, and can be discharged by circulating hydrogen, or a static drainage method can be used. This battery has a start-up time of only 15 minutes and can be stopped instantaneously. More environmentally friendly than lithium iron phosphate batteries.
1, the material is cheap
2, easy to operate
3, security
4, efficient
5, no pollution
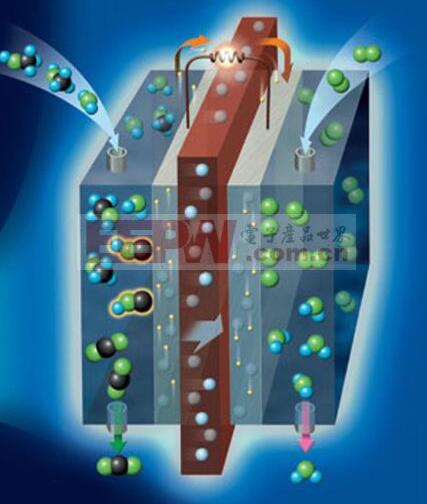
Hydrogen-oxygen fuel cell working principle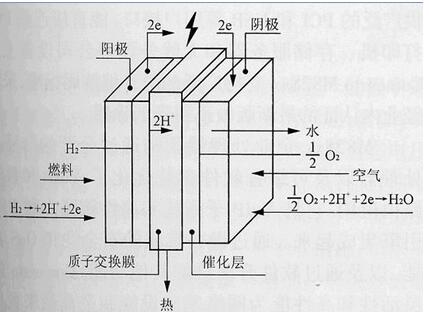
A fuel cell is a chemical battery that uses the energy released by a chemical reaction of a substance to directly convert it into electrical energy. From this point of view, it is similar to other chemical batteries such as manganese dry batteries, lead batteries, and the like. However, it requires continuous supply of reactive materials - fuel and oxidant - which is not the same as other common chemical batteries. Since it converts the energy released by the chemical reaction into a power output, it is called a fuel cell. Specifically, a fuel cell is a "generator" that utilizes the reverse reaction of electrolysis of water. It consists of a positive electrode, a negative electrode and an electrolyte plate sandwiched between the positive and negative electrodes. Initially, electrolyte plates were formed by infiltration of electrolyte into porous plates and are now being developed into electrolytes that use solids directly.
During operation, fuel (hydrogen) is supplied to the negative electrode, and an oxidant (air, which acts as oxygen) is supplied to the positive electrode. Hydrogen is decomposed in the negative electrode into positive ions H+ and electrons e-. Hydrogen ions enter the electrolyte, and electrons move along the external circuit to the positive electrode. The electrical load is connected to an external circuit. On the positive electrode, oxygen in the air and hydrogen ions in the electrolyte absorb electrons reaching the positive electrode to form water. This is the reverse of the electrolytic reaction of water.
Using this principle, the fuel cell can continuously transmit electricity to the outside during operation, so it can also be called a "generator."
In general, writing the chemical reaction equation of a fuel cell requires a high degree of attention to the acidity and alkalinity of the electrolyte. The electrode reaction occurring on the positive and negative electrodes is not isolated, and it is often closely related to the electrolyte solution. For example, hydrogen-oxygen fuel cells are available in both acid and basic forms. In the acid solution, the reaction formula of the negative electrode is: 2H2-4e-==4H+ The positive electrode reaction formula is: O2 + 4H+ + 4eˉ == 2H2O; if it is in an alkali solution , it is impossible to have H+, and in the acid solution, OHˉ cannot occur.
If the electrolyte solution is an alkali or a salt solution, the reaction formula of the negative electrode is: 2H2 + 4OHˉ-4eˉ== 4H20 The positive electrode is: O2 + 2H2O + 4eˉ== 4OHˉ
If the electrolyte solution is an acid solution, the reaction formula of the negative electrode is: 2H2-4eˉ=4H+ (cation), and the positive electrode is: O2+4eˉ+4H+=2H2O
The law of memory is as follows:
Under alkaline conditions, it is easy to remember that the coefficients in front of O2, H2O, eˉ, and 4OHˉ of the positive electrode are 1, 2, 2, and 4, respectively, and then subtract the above formula from the total reaction equation. Under acidic conditions, it is easy to remember the negative reaction formula (4H+)+(-4eˉ)=2H2. The desired equation can be obtained by shifting the term, and the positive reaction formula can be obtained by subtracting the total reaction formula.
Hydrogen oxygen fuel cell
A battery that uses hydrogen as a fuel and oxygen as an oxidant to convert chemical energy into electrical energy through a combustion reaction of the fuel.
When the oxyhydrogen fuel cell operates, hydrogen is supplied to the hydrogen electrode while oxygen is supplied to the oxygen electrode. Hydrogen and oxygen form water through the electrolyte under the action of a catalyst on the electrode. At this time, there is excess electrons on the hydrogen electrode and negatively charged, and the oxygen electrode is positively charged due to lack of electrons. This process similar to combustion can be carried out continuously after the circuit is switched on.
Electrode reactionHydrogen-oxygen fuel cell (neutral medium):
Positive electrode: O2 + 2H2O + 4e- → 4OH-
Negative electrode: 2H2 - 4e- → 4H+
Total reaction formula: 2H2 + O2 == 2H2O
Hydrogen-oxygen fuel cell (acid medium):
Positive electrode: O2 + 4H+ + 4e- → 2H2O
Negative electrode: 2H2 - 4e-→ 4H+
Total reaction formula: 2H2 + O2 == 2H2O
Hydrogen-oxygen fuel cell (alkaline medium):
Positive electrode: O2 + 2H2O + 4e- → 4OH-
Negative electrode: 2H2 - 4e- + 4OH- → 4H2O
Total reaction formula: 2H2 + O2 == 2H2O
Rotary encoders are used as sensors for angle,position,speed and acceleration. We can offer incremental encoders and absolute encoders.

Absolute Encoder,Custom Encoder On Motor,Custom Optical Encoders,High Resolution Encoder
Yuheng Optics Co., Ltd.(Changchun) , https://www.yhenoptics.com